This Is What Happens When We Age
(and What Actually Helps Slow It Down)
Aging can feel mysterious. Your energy changes. Muscles act differently. Sleep becomes harder. And sometimes you just don’t feel like the younger version of yourself anymore. But aging isn’t magic — it’s biology. Most of it comes down to how your cells work, how well they keep themselves clean, and whether your body has enough energy to repair daily wear and tear.
Let’s walk through that process together. I’ll keep the science simple, the explanations clear, and yes — I’ll include a few laughs, because aging is easier to face when we understand it.
Mitochondria: The Tiny Power Plants That Run Your Life
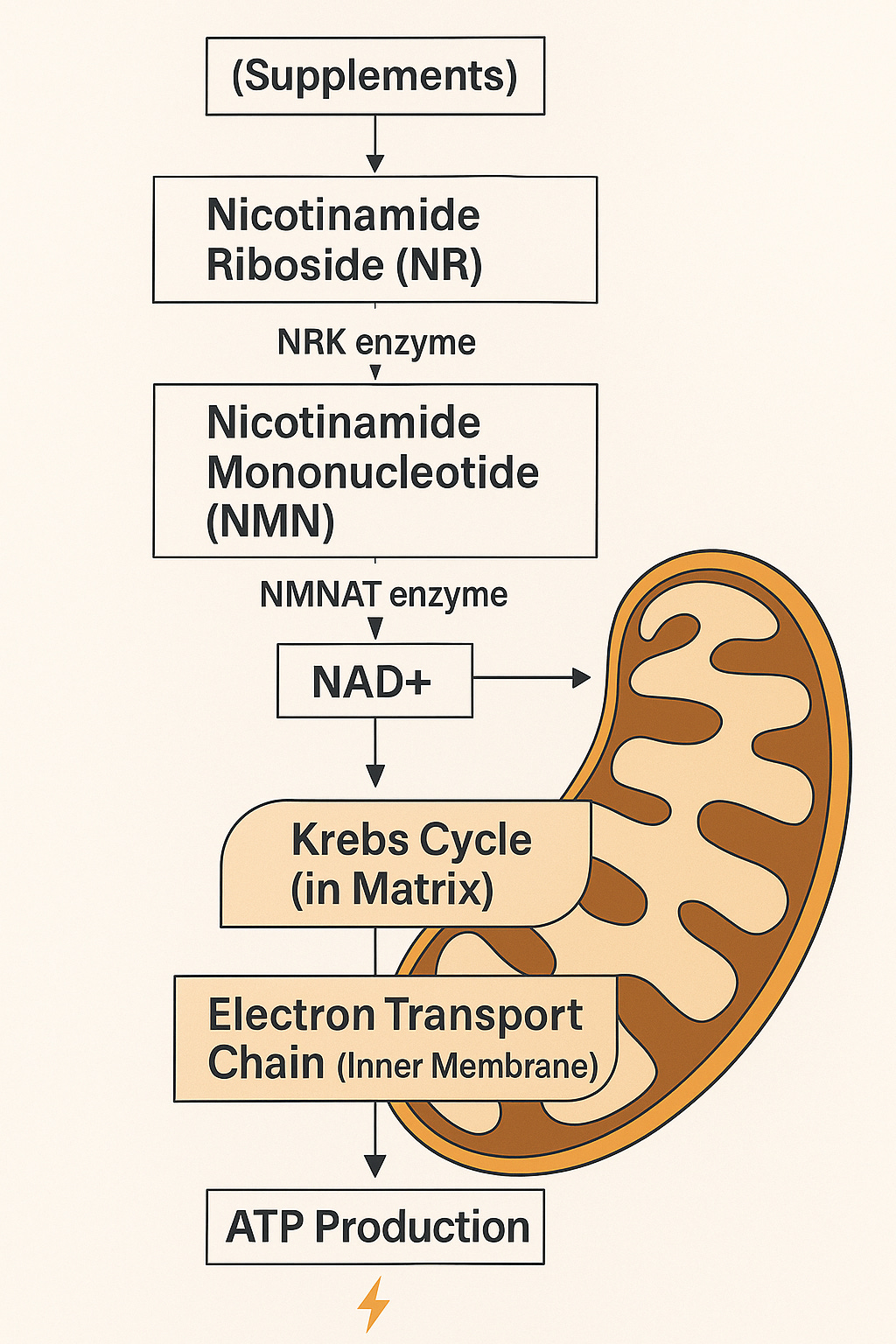
Deep inside nearly every cell in your body are small structures called mitochondria. These tiny units are often described as the “power plants” of the cell, but that nickname undersells how important they truly are.
Mitochondria creates more than 90% of the energy your body uses. They turn the food you eat and the oxygen you breathe into a usable fuel called ATP. When they work well, you feel strong, clear-headed, and active. When they slow down, you feel tired, stiff, foggy — and older.
A Little Cell Inside the Cell
Here’s the wild part: mitochondria started their existence as bacteria. About 1.5 billion years ago, a simple cell swallowed a smaller one. Instead of digesting it, they formed a permanent partnership. That tiny visitor became the mitochondrion. The host cell became the first complex cell.
You still carry that history inside you. Mitochondria have their own DNA, their own membranes, their own ribosomes, and their own way of dividing. They are tiny reminders of the ancient teamwork that made complex life possible.
How They Make Energy
Your mitochondria run two major systems inside their walls: the Krebs Cycle and the Electron Transport Chain. Together, these systems create ATP — the fuel for everything your body does.
Mitochondrial Exhaust
Along the way, mitochondria produce “exhaust”—reactive oxygen species. Young mitochondria handle this easily. Older mitochondria leak more exhaust and repair themselves less often. That leads to inflammation, fatigue, and faster aging.
The Cleanup Crew
Your cells use a system called mitophagy to recycle worn-out mitochondria. As we age, mitophagy slows. Broken mitochondria pile up like clutter in a garage.
Before We Go Further… A Quick Reality Check From Human History
For most of human history, people didn’t live long enough to worry about aging pathways. You didn’t get the luxury of thinking about mitochondria or NAD when bad teeth, contaminated water, infections, childbirth, injuries, or simple accidents could take your life early. Now that many of those threats have been reduced, we finally have the privilege — and responsibility — to understand aging itself. I’ll explore this history in a future post.
The RFK Jr. Moment: “Mitochondrially Challenged Kids”
Recently, RFK Jr. said he can spot kids in airports who are “mitochondrially challenged.”
Let’s pause there.
This is not how medicine works. You cannot diagnose mitochondrial problems by watching a kid walk through Terminal 4.
Real childhood mitochondrial diseases are rare, genetic, severe, and diagnosed with advanced testing. A tired or clumsy child in an airport is… a tired or clumsy child in an airport.
Actual mitochondrial decline largely affects adults, through stress, aging, inflammation, chronic disease, and inactivity — not because someone looked at a stranger near the baggage carousel and simply “felt” their mitochondria were off.
If we’re going to talk about mitochondria, we need science, not soundbites.
Senescent Cells: When Cells Retire, but Don’t Leave
As we age, some cells stop dividing and become senescent. They don’t die, but they release inflammatory chemicals called SASP. These molecules irritate nearby cells, weaken tissues, and speed up aging.
Too many senescent cells are linked to slower healing, more inflammation, muscle loss, and fatigue. They’re like grumpy neighbors who refuse to move and won’t stop yelling.
NAD: The Fuel for Repair
NAD is a molecule your cells use for DNA repair, energy production, stress response, and mitophagy. Unfortunately, NAD levels drop each decade. Lower NAD means slower repair and more damage. That’s why NAD, NR, NMN, and other precursors keep showing up in conversations about aging — the biology is real, even if the marketing gets ahead of the data.
Calorie Restriction vs Fasting
People love to argue whether calorie restriction or fasting is “better” for aging.
Here’s the truth: We don’t have strong human evidence that one is superior.
Both approaches lower inflammation, reduce insulin resistance, support cellular cleanup, and help with weight control. What matters is which pattern keeps your metabolism healthy and your lifestyle sustainable.
Keto, Carnivore, and the “No Sugar = Anti-Aging” Myth
Keto and carnivore communities sometimes claim that removing sugar “reverses aging.” But biology isn’t that simple.
Your body always makes glucose — even if you eat zero carbs.
These diets can help certain people improve metabolic health, but there is no clinical trial showing keto or carnivore slow aging, reduce senescent cells, improve NAD long-term, or increase lifespan. They are tools, not miracles.
Medications That Protect Healthspan
While we don’t have an “anti-aging drug,” we do have medications that protect people from the diseases that shorten life.
These include:
• Osteoporosis medications to prevent fractures
• Statins to reduce heart attacks and strokes
• Blood pressure medications to protect your brain and heart
• GLP-1 drugs that improve metabolic health and inflammation
• Metformin for diabetes (still unproven for longevity in healthy adults)
Longevity often depends on preventing the things that take life early.
Sleep: The Quiet Key to Aging Well
Good sleep is one of the strongest anti-aging strategies on Earth. While you sleep, your body repairs mitochondria, resets hormones, clears brain toxins, reduces inflammation, and restores immune function.
Poor sleep does the opposite. It speeds up aging from the inside out.
Hormones: Menopause, Testosterone, and Healthspan
Hormone levels shift as we age. Women experience menopause; some men develop age-related low testosterone.
Hormone therapy can improve sleep, boost energy, help mood, and maintain bone strength. But it does not extend lifespan. It improves quality of life, not quantity.
We’ll explore this more in a future post.
Scams, Peptides, and the Shirtless Influencer Problem
Aging attracts scammers.
Be cautious of unregulated hormone blends, online “longevity clinics,” illegal peptide injections, testosterone sold to men with normal levels, miracle diets, growth hormone “boosters,” and 20-year-olds selling “anti-aging hacks” with an affiliate code.
Protein helps with muscle — but only if you use the muscle.
Peptides don’t reverse aging.
Supplements can’t replace science.
What Actually Slows Aging
The most effective tools are beautifully boring:
• resistance training
• daily movement
• quality sleep
• Mediterranean-style eating
• strong metabolic health
• low LDL
• stress control
• treating conditions early
• targeted supplements with evidence
You don’t need magic. You need consistency.
Join Us for the Mediterranean Longevity Cruise
We’re taking these ideas off the page and onto the water.
Later this year, we’ll be hosting a Mediterranean Longevity Cruise, where we’ll cover what actually helps you age well — what science supports, what’s nonsense, and how to live longer and better.
We’ll explore regions that inspired longevity research, including coastal towns that helped shape the original Blue Zones concept.
And don’t worry: we are not forcing you to hike with a rucksack. If we go on a walk, it will be because the gelato shop is at the top of a hill.
More details soon — but if you want real science, real food, and fun, you’ll want to join us.
Coming Up in the Paid Section
For subscribers, I’ll dig deeper into:
• the science of NAD and repair
• how mitochondria fail with age
• how mitophagy works
• senescent cell pathways
• the truth about “longevity molecules”
• the evidence behind Urolithin A, NMN, NR, and rapamycin
• how menopause and testosterone changes really affect aging
• how exercise builds new mitochondria
• what supplements are worth your time
• and how to spot anti-aging scams instantly
Science, not superstition.
Evidence, not influencers.
Healthspan, not hype.
Thanks for supporting this work. Below is the deeper layer — the part where we move past the headlines, past the influencer shortcuts, and into the biology that actually matters.
Let’s take a closer look at what aging really is, how it unfolds, and what science (not marketing) says about slowing it.
🔬 1. How Mitochondria Fail with Age
Mitochondria lose function for several reasons, and each one accelerates aging in a slightly different way.
A. Mutations in Mitochondrial DNA (mtDNA)
Unlike the DNA in your nucleus, mitochondrial DNA has:
no protective histones
limited repair mechanisms
constant exposure to free radicals from energy production
As damage accumulates:
ATP production falls
oxidative stress rises
mitochondria become less efficient
defective mitochondria begin to dominate
This becomes a vicious cycle:
more damage → less energy → more inflammation → more damage.
B. Decline in Mitophagy
Young cells clear damaged mitochondria quickly. Older cells do not.
Mitophagy requires:
PINK1
Parkin
lysosomal enzymes
intact mitochondrial membranes
adequate NAD levels
In aging tissue, interruptions in any of these steps allow defective mitochondria to build up.
C. Mitochondrial Network Fragmentation
Healthy mitochondria fuse and divide constantly. With age, fusion proteins decrease. This causes fragmentation — tiny isolated mitochondria that don’t work well.
Fragmented mitochondria:
produce more ROS
generate less ATP
trigger inflammatory pathways
This is one of the clearest markers of cellular aging.
🧬 2. NAD: The Repair Currency of the Cell
NAD is required for:
DNA repair (via PARPs)
activation of sirtuins (SIRT1, SIRT3)
metabolic flexibility
mitophagy
proper circadian rhythm
stress resistance
Why NAD drops with age
Increased PARP activation from DNA damage drains NAD
Reduced NAMPT slows NAD recycling
Poor sleep disrupts NAD rhythms
Chronic inflammation diverts NAD to repair
Mitochondrial decline slows NAD generation
NAD precursors: what we know
NR and NMN reliably raise NAD levels in humans.
UA indirectly supports NAD by improving mitochondrial quality.
But we still lack:
long-term safety data
hard clinical aging endpoints
clear dosing guidelines
The biology is plausible.
The marketing is premature.
🧓 3. Senescent Cells: The Slow-Moving Wildfire
Senescent cells are a major driver of aging. They:
stop dividing
resist apoptosis (cell death)
secrete inflammatory SASP
damage surrounding tissue
alter stem cell behavior
disrupt mitochondrial function
What pushes cells into senescence?
telomere shortening
oxidative stress
mitochondrial damage
chronic inflammation
DNA damage
oncogene activation
environmental toxins
Why senescent cells accumulate with age
immune system declines
mitophagy slows
NAD drops
apoptosis pathways weaken
This combination turns senescent cells into long-term residents.
Can we remove them?
Senolytics (like dasatinib + quercetin) work in mice — but we lack human data.
Senomorphics (like Urolithin A) reduce SASP without killing cells — promising but early.
This field is exciting, but we’re not ready for mainstream clinical use.
🔬 4. Urolithin A: Where It Actually Fits
Urolithin A activates mitophagy. It does so through:
upregulation of PINK1
activation of Parkin
modulation of AMPK
enhancement of autophagosome formation
stabilization of mitochondrial networks
Human trials show:
improved muscle endurance
reductions in inflammation markers
reduced ceramides and acylcarnitines
consistent plasma levels even in “non-producers”
But importantly:
No improvement yet in 6-minute walk distance, VO2 max, or large-scale functional outcomes.
UA is promising, safe, and mechanistically sound — but not proven to change major aging endpoints.
🧠 5. Exercise: The Most Powerful Mitochondrial Intervention on Earth
Nothing — no pill, no peptide, no supplement — does what exercise does.
Resistance training:
activates mTOR in a controlled, anabolic way
increases muscle satellite cells
boosts mitochondrial biogenesis via PGC-1α
improves insulin sensitivity
reduces senescent cell load
raises NAD
enhances mitophagy
Muscle is longevity.
Muscle is metabolism.
Muscle is medicine.
Zone 2 aerobic exercise:
increases mitochondrial density
improves fat oxidation
raises VO2 max
reduces ROS production
enhances metabolic flexibility
If exercise were a drug, it would be the most effective and profitable drug in history.
🌙 6. Sleep: The Cellular Reset Button
Sleep regulates:
NAD synthesis
mitochondrial turnover
DNA repair
glymphatic brain cleanup
immunologic recalibration
cortisol and insulin balance
Short sleep disrupts:
sirtuin activity
metabolic switching
memory consolidation
hormonal cycling
cellular repair
Sleep is not optional.
It is the nightly “anti-aging treatment” we were designed to use.
🍽 7. Diet: What Actually Supports Longevity Pathways
Forget superfoods and influencer stacks. The patterns that work are simple:
The Mediterranean pattern improves:
insulin sensitivity
LDL
inflammation
microbiome diversity
mitochondrial efficiency
Plant polyphenols do real work:
increase AMPK
support autophagy
protect mitochondria
nourish beneficial microbes
Protein + movement builds muscle:
But protein without resistance training becomes calories, not muscle.
Ultra-processed foods harm:
mitochondria
insulin signaling
NAD recycling
stem cell pools
Keto/carnivore clarity:
They help some people lose weight and reduce insulin — indirectly improving aging pathways.
There is zero human evidence they slow aging independently of weight loss.
💊 8. Medications: Where They Fit Into Aging Biology
Osteoporosis drugs
Prevent fractures — one of the most age-defining turning points.
Statins
Reduce inflammation, stabilize plaques, and prevent the diseases that kill seniors.
GLP-1 agonists
Improve insulin resistance, inflammation, and cardiovascular risk.
Metformin
Interesting mechanisms (AMPK, IGF-1 modulation), but no proof yet of longevity benefit in healthy adults.
Rapamycin
Promising in animals.
Risky in humans outside clinical supervision.
Trials are underway.
We are not at the point where any drug can be responsibly marketed as an “anti-aging therapy.”
🚫 9. The Anti-Aging Marketplace: What to Ignore
Peptides with no clinical trials
“Anti-aging” testosterone in men with normal levels
Growth hormone “boosters”
Unregulated online hormone clinics
Detox powders
Cellular “reboot” injections
Misinformation wrapped in affiliate codes
Aging biology is complex, fragile, and powerful.
It cannot be hacked by a supplement stack designed by someone who still needs their mom’s Netflix password.
💛 10. The Framework That Actually Works
Here is what the best evidence — across human and animal research — supports:
Aging slows when:
you build and maintain muscle
you get enough sleep
you keep LDL low
your blood pressure stays controlled
your weight stays healthy
you move daily
you eat mostly plant-forward whole foods
you avoid tobacco
you manage stress
you treat chronic diseases early
you maintain a healthy social network
And yes, mitochondrial support through UA, NR, NMN, omega-3s, magnesium, and a few other supplements may offer a small, biologically plausible benefit — but none replace the fundamentals.
🧭 Coming Up for Subscribers
In future paid posts, we’ll explore:
• How telomeres work (and why supplements won’t fix them)
• The truth about NAD precursors — who benefits, who doesn’t
• Breaking down longevity drugs: rapamycin, acarbose, SGLT2s
• The microbiome and aging
• Fasting-mimicking diets (what the data says)
• Mitochondrial supplements: rating them from best to bogus
• How menopause and andropause change metabolic health
• What really increases healthspan in clinical trials
• The myths and lies of “biohackers”
Science, not superstition.
Evidence, not anecdotes.
Healthspan, not hype.